Introduction: Sickle cell anemia (SCA) results from a mutant β-globin gene that produces abnormal hemoglobin S (HbS). HbS polymerizes upon deoxygenation, resulting in red blood cell (RBC) sickling and membrane damage, leading to vaso-occlusions and hemolysis. Additionally, sickle RBCs contain less ATP and more 2,3-diphosphoglycerate (2,3-DPG) than normal RBCs; 2,3,DPG allosterically reduces hemoglobin (Hb) oxygen (O2)-affinity [i.e. increases P50], promoting faster unloading of O2, which potentiates HbS polymerization and RBC sickling. FT-4202, a selective and orally bioavailable allosteric activator of RBC pyruvate kinase (PKR), decreases 2,3-DPG and increases ATP in normal human RBCs (Blood, 2019, 134, Supplement 1:616). We hypothesized that oral administration of FT-4202 to SCA mice will increase HbS O2-affinity, and thereby decrease RBC sickling and membrane damage.
Methods: Berkeley SCA mice were given 500-1000 mg/kg/day FT-4202 in chow (FT-4202 group) or control chow (control group) in 4 cohorts for 2 weeks (total 17-18 mice/group). In all cohorts, the health status, weight, and average chow consumption of each mouse was determined 3 times/week. Three cohorts were injected with sulfo-NHS-biotin 1 week into treatment (10-11 mice/group), and RBC survival assessed over the next week with serial micro-bleeds while on treatment. The 4th cohort was only bled at 2 week time-point to obtain P50 (Hemox Analyzer) and Hb levels (Hemavet). At experiment termination, all cohorts were terminally bled to determine (a) RBC levels of 2,3-DPG and ATP, (c) plasma levels of FT-4202 by LC-MS/MS, (d) the proportion of irreversibly sickled RBC (ISC) on blood smears (Image-J analysis), (e) the kinetics of experimentally-induced sickling (Lorrca®Oxygenscan) and (f) membrane deformability (Lorrca®Ektacytometry).
Results: SCA mice on FT-4202 consumed a similar amount of food, and had similar weights and survival, compared to SCA mice on control chow throughout the 2-week period. As hypothesized, HbS O2 affinity increased, reflected by a decrease in P50 from 29.6 ± 0.62 mmHg (mean ± SEM) in the control group to 27.6 ± 0.58 mmHg in the FT-4202 group (p<0.03). Determinations of 2,3-DPG, ATP and FT-4202 are ongoing and will be presented.
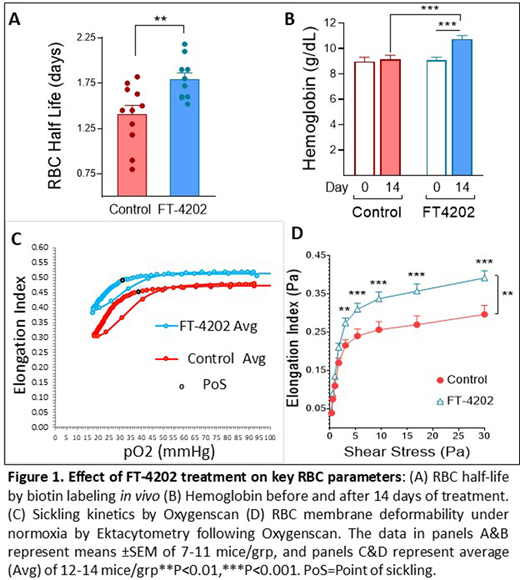
As expected, this increased HbS O2-affinity in the FT-4202 group reduced RBC sickling and membrane damage. At 2 weeks, the proportion of ISCs on blood smears was reduced in the FT-4202 group to 2.4 ± 0.3% vs. 5.9 ± 1.4% in the control group (p<0.02). The sickle RBC half-life increased to 1.8 ± 0.07 days in FT-4202 group vs. 1.4 ± 0.1 days in the control group, a 28% increase in RBC survival (p<0.01, Figure 1A). Hence, Hb levels in the FT-4202 group increased from 9.1 ± 0.2 g/dL before treatment, to 10.8 ± 0.3 g/dL 2 weeks after treatment (p<0.001), while Hb levels in the control group remained unchanged (Figure 1B). The reticulocytes remained unchanged in both groups before and after treatment.
When sickle RBCs were de-oxygenated from an ambient pO2 of ~150 mmHg to a pO2 of 10-15 mmHg, followed by their re-oxygenation to ambient pO2 at a constant shear stress of 30 Pa (Oxygenscan), the point of sickling (PoS; pO2 level when the EI becomes 95% of the EI at ambient O2) decreased on average from 37% pO2 in the control group, to 30% pO2 in the FT-4202 group (p<0.002, Figure 1C), with a significantly improved Elongation Index at the point of minimum pO2 (EImin), (p<0.05). Next, RBC membrane deformability was measured under ambient pO2 (normoxic conditions), but varying shear stress after the de-oxygenation/re-oxygenation cycle on the Oxygenscan. Sickle RBCs from the FT-4202 group were significantly more deformable [i.e. had a higher Elongation Index (EI)] compared to control sickle RBCs (p<0.01, Figure 1D), as shear stress increased to ≥3 Pa, demonstrating that FT-4202 sickle RBCs sustained significantly less membrane damage following sickling and un-sickling.
Conclusion: A 2-week oral FT-4202 administration was well tolerated by SCA mice and demonstrated beneficial biological effects: improved RBC membrane deformability and sickling parameters, with a shift in the PoS to lower pO2, and increased RBC survival and Hb levels. A parallel human phase-I study in healthy subjects and sickle cell disease patients to assess the safety and PK/PD of FT-4202 is ongoing (NCT03815695). Overall, our results suggest that FT-4202 can be a potentially useful orally available agent with significant anti-sickling effect.
Drake:Forma Therapeutics: Other: Shareholder of Forma Therapeutics. Fulzele:FORMA Therapeutics, Inc: Current Employment, Other: Shareholder of Forma Therapeutics. Guichard:FORMA Therapeutics, Inc: Current Employment, Other: Shareholder of Forma Therapeutics; AstraZeneca: Other: Shareholder. Malik:Aruvant Sciences, Forma Therapeutics, Inc.: Consultancy; Aruvant Sciences, CSL Behring: Patents & Royalties.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal